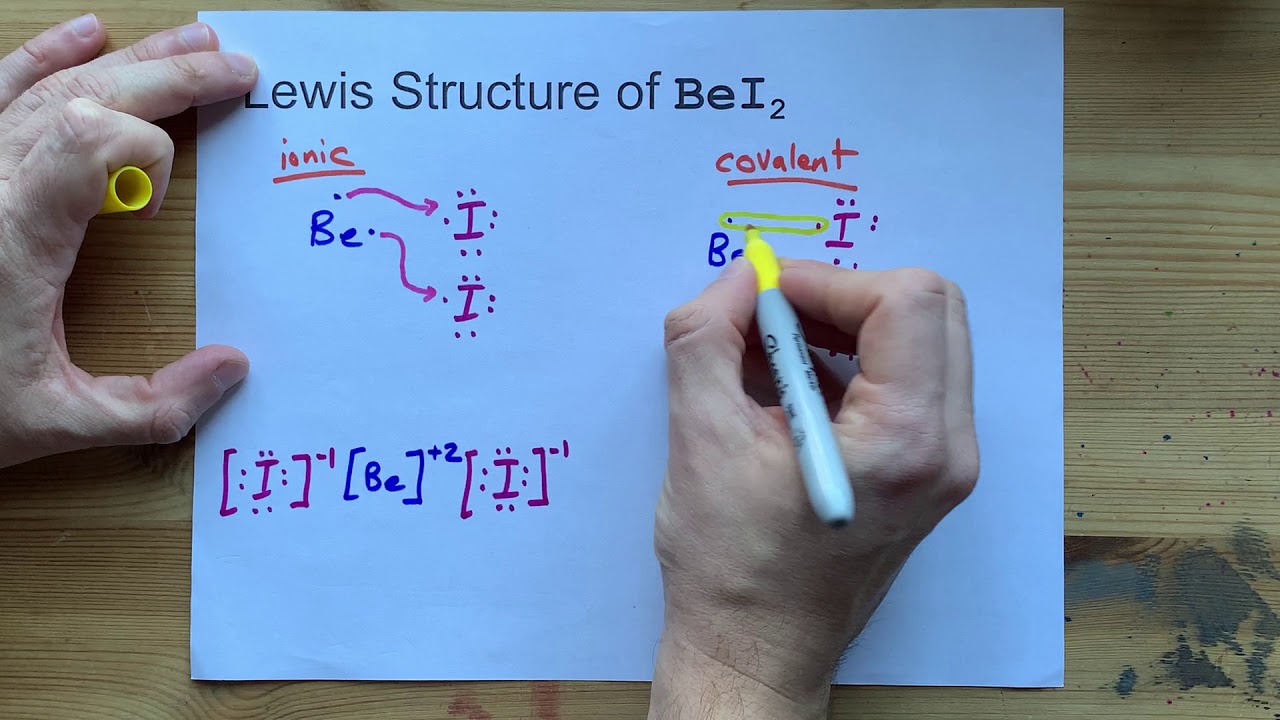
What Is the Chemical Formula for Beryllium and Iodine
The molecular formula of potassium iodide is KI Potassium has an atomic weight 390983 and Iodine has an atomic weight 12690447 adding both of them we get. Be I 2 BeI 2.
Beryllium Iodide Bei2 Chemspider
Part B beryllium and iodine Express your answer as a chemical formula.

. It reacts with fluorine giving beryllium fluoride and fluorides of iodine with chlorine giving beryllium fluoride and with bromine giving beryllium bromide. Beryllium iodide is also formed when beryllium carbide reacts with hydrogen iodide in the gas phase. Beryllium iodide is also formed when beryllium carbide with hydrogen iodide in the gas phase.
Iodine atoms achieve one electron to turn into the. The iodine atom has a radius of 140 pm and a Van der Waals radius of 198 pm. Be 2 C 4 HI 2 BeI 2 CH 4.
See more Iodine products. Potassium iodide is made of ions such as K I and behaves like an ionic salt. The nucleus is composed of protons and neutrons.
What is the chemical formula for sodium sulfide. Potassium iodine formula are K and I respectively 12690447 390983 1660028. How many oxidation number does fluorine have.
The chemical symbol for Beryllium is Be. ΑΣφ h t o. What is the formula for strontium.
Beryllium Iodide has 1 atom of Beryllium and 2 atoms of Iodine. 7 5 3 1 and -1. In compounds of iodine where known the most common oxidation numbers of iodine are.
The chemical formula for table salt otherwise known as sodium chloride is NaCl. Calculation of Molar Mass. The heaviest of the stable halogens it exists as a semi-lustrous non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius and boils to a violet gas at 184 degrees CelsiusThe element was discovered by the French chemist Bernard Courtois in 1811 and was named two.
Be 2 C 4 HI 2 BeI 2 CH 4. What is the chemical name for P₄S₉. Beryllium Iodide molecular weight.
Chemical Symbol for Iodine. Beryllium iodide is also formed when beryllium carbide reacts with hydrogen iodide in the gas phase. Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structure.
The iodine in beryllium iodide is easily replaced with the other halogens. Since there is a tendency to gain or lose electrons to achieve a stable state we get the formula KI. 9012182 126904472 Percent composition by element.
Scientists researching compounds present at low temperatures created disulfur dioxide. Just so what is the chemical formula for the compound shaped between strontium and iodine. Which element has the same oxidation number in all of its compounds.
Be I 2 BeI 2. Iodine is a chemical element with the symbol I and atomic number 53. Potassium Iodide Chemical Formula.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Strontium ion Sr2 PubChem. Use subscripts to represent the number of each type of atom or ion in a molecule.
Strontium atoms lose two electrons to turn into a cation Sr2. Iodine is the heaviest of the stable halogens it exists as a lustrous purple-black metallic solid at standard conditions that sublimes readily to form a violet gas. What is Lithiums oxidation number.
Be 2 C 4 HI 2 BeI 2 CH 4. As they are ionically bonded together potassium has an oxidation number of 1 and iodide is -1. Beryllium iodide can be prepared by reacting beryllium metal with elemental iodine at temperatures of 500 C to 700 C.
Be 2 C 4 HI 2 BeI 2 CH 4. The formula for strontium iodide is SrI2. Molar mass of BeI2 262821122 gmol.
Be I 2 BeI 2. The iodine in beryllium iodide is easily replaced with the other halogens. This in combination with research into sulfur oxides relating to pollution on Earth has led to renewed interest in sulfur oxide compounds.
Beryllium iodide reacts with fluorine giving beryllium fluoride and fluorides of iodine with chlorine giving beryllium chloride and with bromine giving beryllium bromide. 53 is a Block P Group 17 Period 5 element with an atomic radius of 12690447. What is the chemical formula for Calcium Bromide.
Potassium Iodide can be produced in industries by treating KOH with iodine. Convert grams Beryllium Iodide to moles or moles Beryllium Iodide to grams. Write the chemical formula for this compound.
What is Iodines oxidation number. Beryllium iodide is also formed when beryllium carbide with hydrogen iodide in the gas phase. Beryllium iodide can be prepared by reacting beryllium metal with elemental iodine at temperatures of 500C to 700C.
What is Berylliums oxidation number. Atomic Number of Beryllium. ха x a 5 1 5 x A chemical reaction does not occur for this question.
What is the chemical formula for magnesium phosphide. BeI2 is the correct formula for this compound. The chemical formula of sucrose is C12H22O11 meaning that each molecule of sucrose has 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms.
Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structureThe chemical symbol for Iodine is I. What is the chemical formula for beryllium fluoride. The number of electrons in each of Iodines shells is 2 8 18 18 7 and its electron configuration is Kr 4d 10 5s 2 5p 5.

Vektor Stok Beryllium Iodide Bei2 Molecule Simple Molecular Tanpa Royalti 1946544133
Belum ada Komentar untuk "What Is the Chemical Formula for Beryllium and Iodine"
Posting Komentar